Like their name implies, biosimilars are similar to originator biologics but not identical.
In fact, because biologic and biosimilar medicines are created from living cell lines, no two medicines are ever exactly the same. To make informed choices, patients, doctors, and health care providers should understand the complexity of these drugs.
“As biosimilars are similar but not identical to the innovator drug, they may not behave in the same way. As such, physicians need to be well-informed regarding any therapeutic changes whether between innovator biologics or innovator and biosimilars biologics.” – Dr. Denis Choquette, Rheumatologist and Professor of Medicine from the University of Montreal
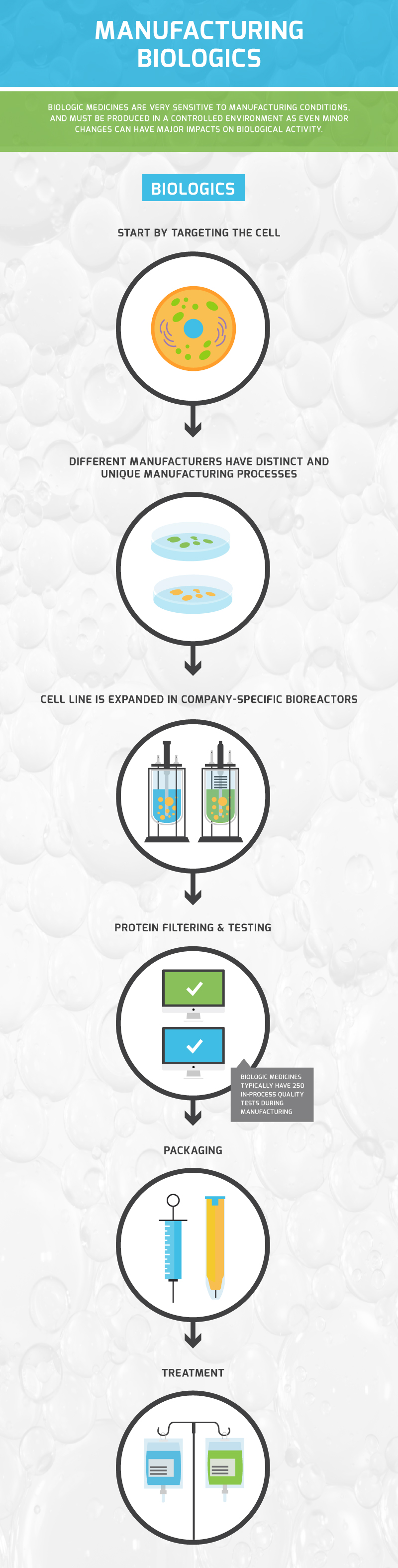
Both biologics and biosimilars require high levels of manufacturing expertise, cutting-edge equipment and are evaluated against high standards. These medicines are highly sensitive, and undergo rigorous quality control measures to ensure purity, safety and efficacy of the product.
See the infographic below for a high-level snapshot of the manufacturing process: