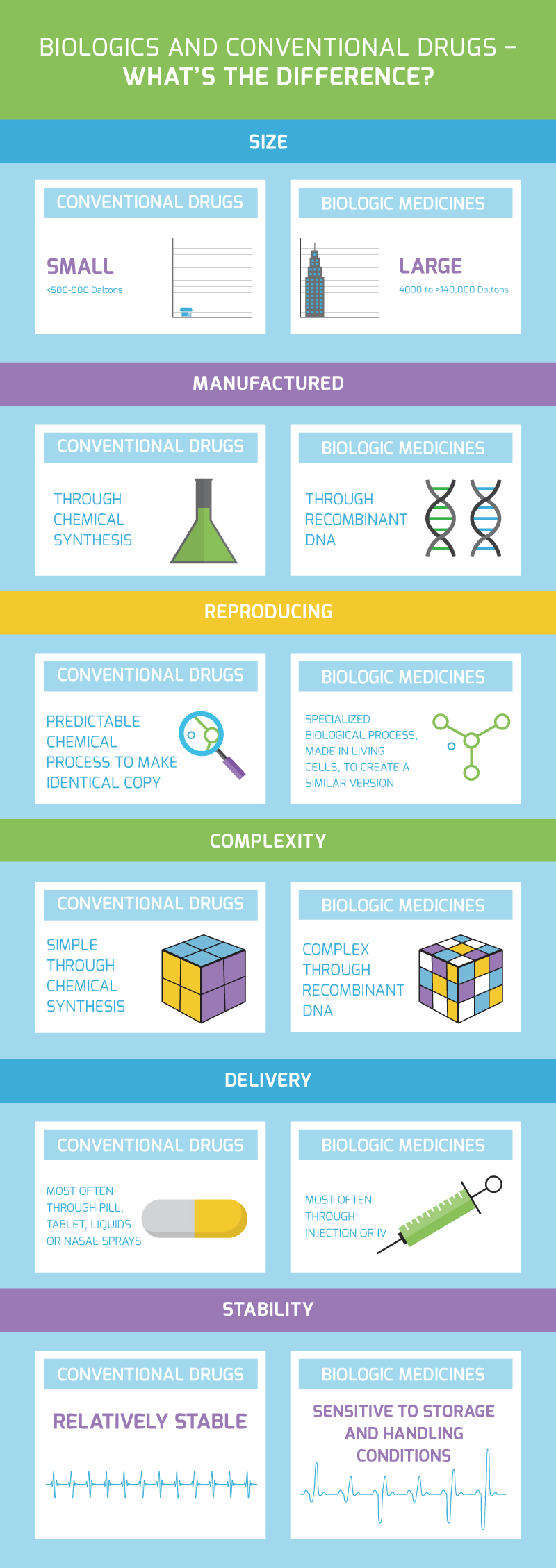
Drugs can be categorized as being synthetically or biologically produced.
Synthetic (conventional) therapies are made from chemical processes — like mixing, heating and cooling — to create the active ingredient compound in a traditional lab. Synthetic drugs such as Aspirin® (acetylsalicylic acid), for example, are created with chemical ingredients and are sometimes referred to as “small molecules.”
A biologic, on the other hand, is defined as a drug derived from living cells. Unlike small-molecule drugs, biologics are large, structurally complex proteins that are not chemically manufactured.
“A good example is insulin, considered to be the first biologic. It’s made by bacteria, implanted with a human gene. The bacteria effectively become little factories, churning out the insulin protein diabetics can use.” – Dr. Leigh Revers, senior lecturer in biotechnology at the University of Toronto
In fact, biologics involve complex manufacturing requirements and specialized processes that do not always resemble the facilities, machinery, or equipment used to produce chemical drugs. Manufactured in living cells, this fragile process must be controlled with utmost precision, as any variation in the development can alter the medicine’s ability to act in the body as intended.
See a visual snapshot below of some of the ways biologics and conventional drugs differ.